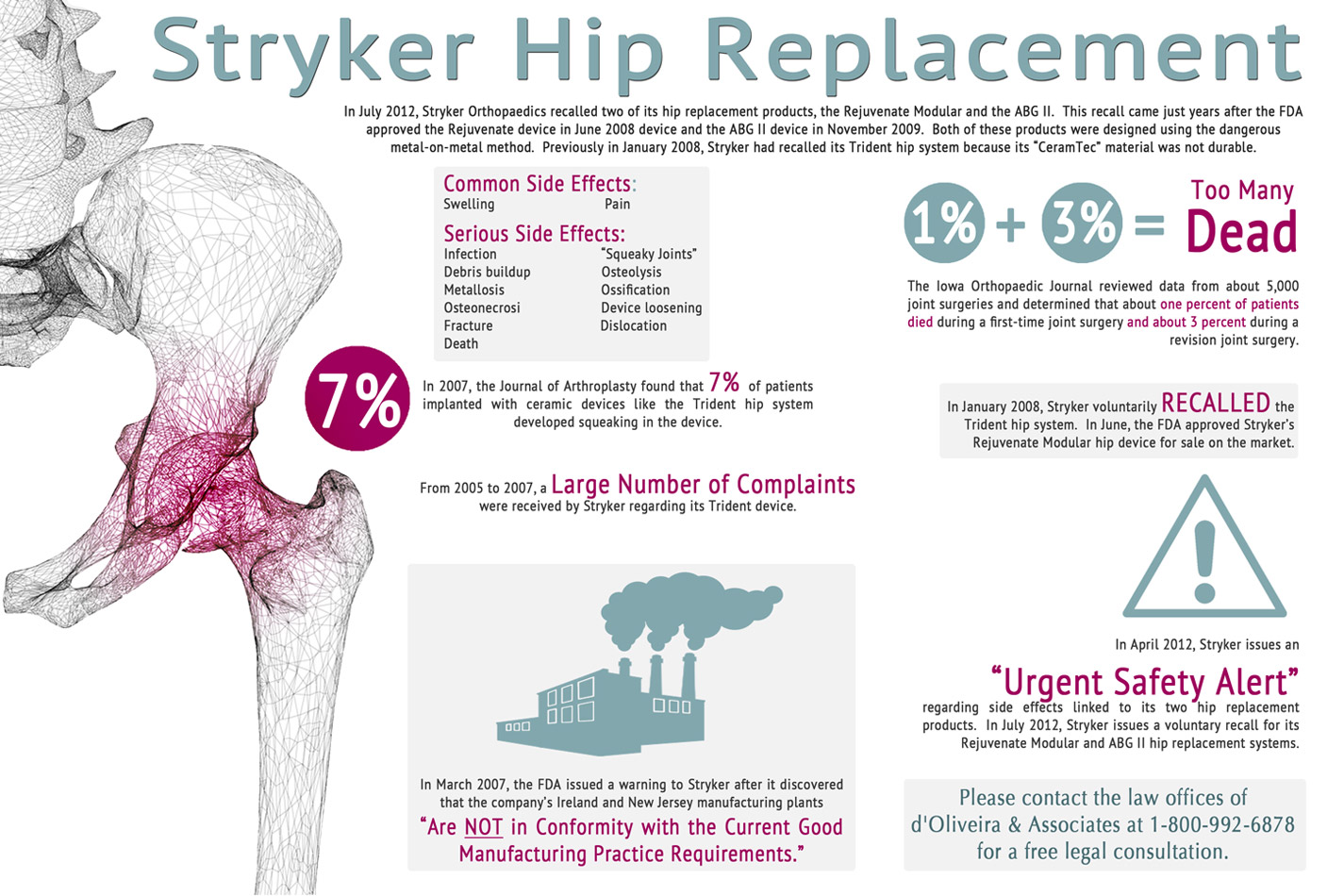
 On November 28, 2007, Stryker Corp., one of the world’s leading manufacturers of hip and other joint replacements was given a warning from the Food and Drug Administration (FDA) regarding their manufacturing facility in Mahwah, New Jersey. According to the letter, the FDA conducted an inspection of the facility and found substandard conditions. This marked the second time that the company had been warned about deplorable conditions in their plants. Stryker in a response letter stated that they did not believe that these conditions posed a threat to patients.
On November 28, 2007, Stryker Corp., one of the world’s leading manufacturers of hip and other joint replacements was given a warning from the Food and Drug Administration (FDA) regarding their manufacturing facility in Mahwah, New Jersey. According to the letter, the FDA conducted an inspection of the facility and found substandard conditions. This marked the second time that the company had been warned about deplorable conditions in their plants. Stryker in a response letter stated that they did not believe that these conditions posed a threat to patients.
The FDA further told Stryker that they had received a number of complaints from patients regarding hip replacement components manufactured at the New Jersey facility. The patients cited implants that did not fit properly leading to problems including pain, difficulty walking, and “squeaky” joints. Others reported that parts of the implant broke off or had been wearing unevenly. Contrary to Stryker’s statements, the FDA letter said that the deficiencies found at the New Jersey plant directly contributed to these defects. The letter also states that Stryker “failed to perform corrective and preventive actions in order to prevent the recurrence of nonconforming product or other quality problems.”
Finally, after numerous warnings and numerous complaints from patients, on January 22, 2008, Stryker recalled two hip implant components made under the company’s Trident line. The recall, surprisingly, was announced in the same letter used to rebut the FDA’s allegations of deplorable conditions at the New Jersey plant. The recall included the Trident Acetabular PSL Cup and the Trident Hemispherical Cups. Stryker says that the recall was implemented amid concerns that the components could be contaminated with “manufacturing residuals” at levels that exceeded company standards.
It is clear that Stryker Corp. had no intention to take the FDA’s warnings seriously. Their failures allowed defective products to be manufactured and these products have caused patients to be injured. How many warnings are required before the FDA steps in and takes action to remove not only defective products from the market, but also rogue companies who think that they make their own rules? A few too many.


