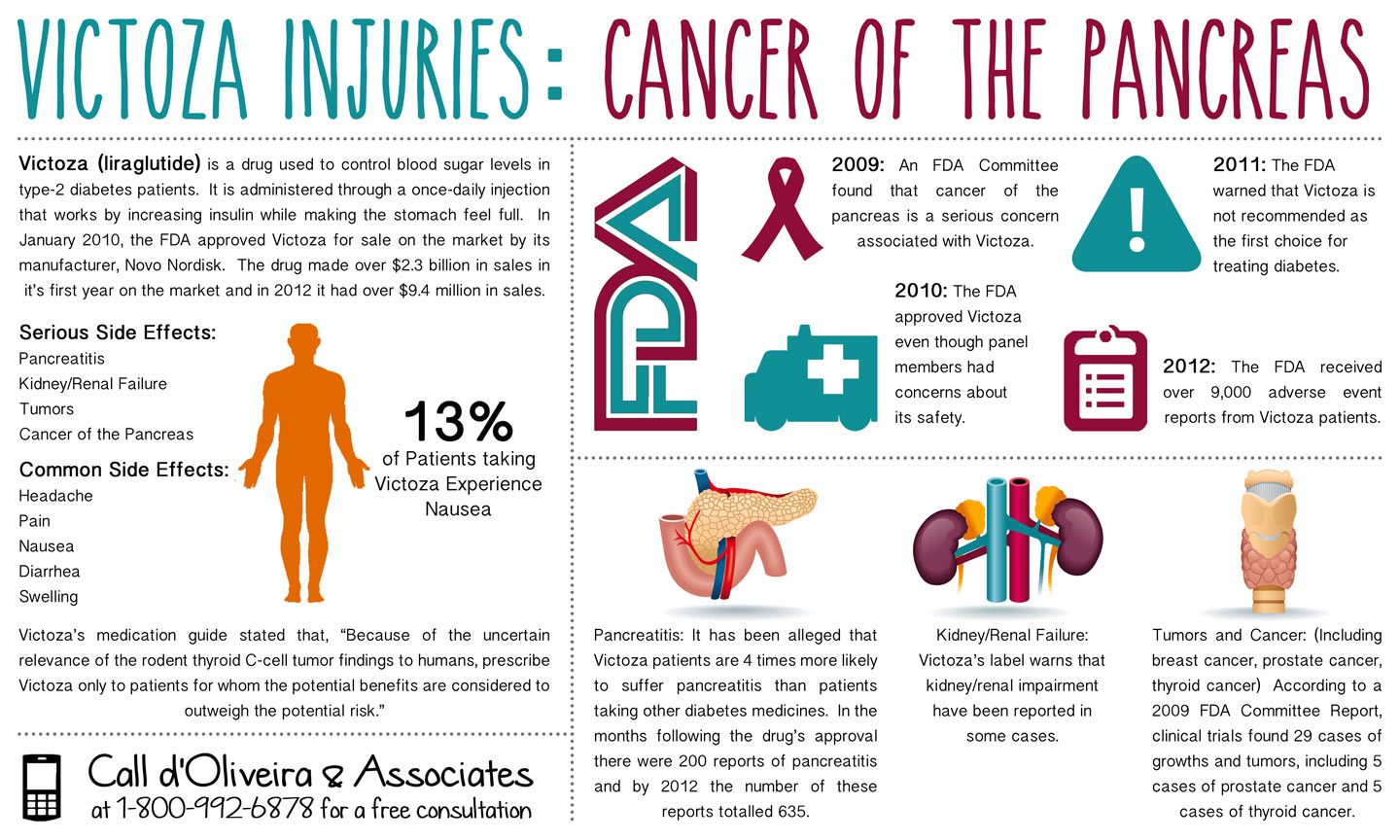
Victoza (liraglutide) is a once-daily injection used to control blood sugar levels in type-2 diabetes patients. This medicine increases insulin while making the stomach feel full. The FDA approved this drug in January 2010 and it earned more than $2.3 billion in sales its first year on the market (i). Victoza, like all medicines, may cause side effects. According to the product’s website, the most common Victoza side effects were headache, nausea, and diarrhea. The manufacturer also warns that this drug may cause low blood sugar, pancreatitis, and cancer (ii). People who have suffered pancreatic cancer or thyroid cancer should talk to a personal injury attorney about their legal options.
As early as 2009 the FDA was concerned about the association between Victoza and cancer. The Agency reviewed the safety of the medicine in April 2009 and discovered twenty-nine cases of growths and tumors, including five cases of prostate cancer and five cases of thyroid cancer (iii). In June 2011, the FDA released new safety information on the drug highlighting the risk of thyroid cancer and acute pancreatitis with Victoza. The Agency also noted that this medicine is not recommended as the first choice for type-2 diabetes patients (iv). The most recent warning came in March 2013 when the FDA warned that the clinical trials had linked the drug to an “increased risk of pancreatitis, or inflammation of the pancreas, and pre-cancerous cellular changes called pancreatic duct metaplasia.” Read the Drug Safety Communication here.
The Victoza infographic provides important information to type-2 diabetes patients, who are using the drug. Patients who have suffered Victoza side effects like cancer may be able to recover damages for medical bills, lost income, pain and suffering, and other injuries. d’Oliveira & Associates has been investigating these cases and the law firm is ready to answer any questions. They also work with some of the more experienced Victoza lawyers, who are handling these cases, and there are no legal fees unless you receive a settlement or award. Contact the law firm online or call 1-800-992-6878 for a free legal consultation.
Source:
- (i) Novo Nordisk 2012 Annual Report.
http://webmedia.novonordisk.com/nncom/images/annual_report/2012/Novo-Nordisk-AR-2012-en.pdf - (ii) Product Website: Understanding Side Effects.
http://www.victoza.com/learn/usage-guidelines.aspx - (iii) Endocrine and Metabolic Drug Advisory Committee, April 2009.
http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM148659.pdf%20FDA%20April%202009 - (iv) Drug Safety Communication, March 2013.
http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm258826.htm