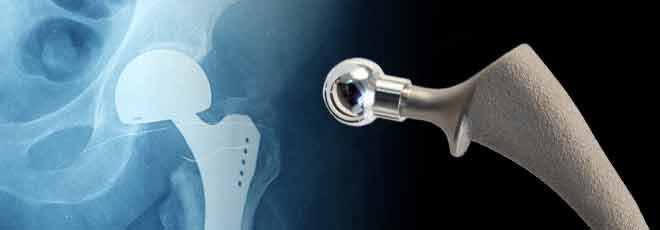
The Stryker LFIT COCR V40 Femoral Head has been recalled due to their serious health risks to patients. You may be entitled to compensation for medical bills, lost income and pain and suffering, among other losses. Our law firm works with some of the more experienced Stryker LFIT COCR V40 Femoral Head lawyers in the country and there is no legal fee unless a settlement or award is obtained. Feel free to contact us toll-free 24/7 at 1-800-992-6878 or submit a contact form online for a free legal consultation.
Have You Suffered an Injury or Complication due to the
LFIT COCR V40 Femoral Head?
Call Us Now For a Free Case Evaluation!
What is a Stryker LFIT COCR V40 Femoral Head?
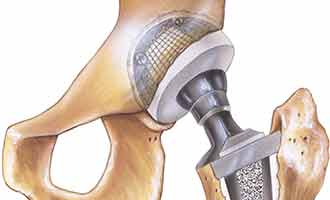 The femoral head used by Stryker is placed on the widely used Accolade TMZF and Accolade 2 stems of the hip implant systems. It is connected to the femoral head portion of the implant with a metal taper lock, to provide overall stability between the two metal pieces. This sophisticated mechanism is intended to allow those who are in need of a new hip to gain comfort and movement back into their lives.
The femoral head used by Stryker is placed on the widely used Accolade TMZF and Accolade 2 stems of the hip implant systems. It is connected to the femoral head portion of the implant with a metal taper lock, to provide overall stability between the two metal pieces. This sophisticated mechanism is intended to allow those who are in need of a new hip to gain comfort and movement back into their lives.
Which Hip Implants Are Being Affected?
| Catalog Number | Head Diameter | Offset |
| 6260-9-236 | 36mm | +5 |
| 6260-9-240 | 40mm | +4 |
| 6260-9-244 | 44mm | +4 |
| 6260-9-340 | 40mm | +8 |
| 6260·9-440 | 40mm | +12 |
| 6260-9-344 | 44mm | +8 |
| 6260-9-444 | 44mm | +12 |
What Are Stryker LFIT COCR V40 Femoral Head Complications?

Serious complications have been identified in connection with the Stryker femoral head components. First, there have been multiple reports concerning failure of the taper lock which holds the femoral head and the femoral neck together. According to The Public Health Agency of Canada (PHAC), Stryker has received more complaints than expected dealing with the taper lock failure of certain sizes of the LFIT COCR C40 femoral heads. When this piece fails, it can lead to severe complications including: loss of flexibility or movement, severe pain, irritation and inflammation, adverse local tissue response, dislocation of hip bones, severe hip and leg joint instability, broken bones, leg length discrepancy due to movement of the joints and the need for comprehensive adjustment surgery.
 Additional complications may occur due to the heavily-criticized metal on metal design of the implant. This design has been criticized due to the metal femoral head and the metal femoral neck rub against each other while in use. This movement can then lead to corrosion of the implant components, ultimately leading to corrective surgeries. Over time, the corrosion of the metal shards by pressing against each other can lead to the metal coursing into the blood stream of the patient who was implanted with a possibly defective Stryker LFIT COCR V40 femoral head. This is life-threatening once metal levels in the blood reach a certain point, leading to possible diagnoses of metallosis or metal poisoning, which can cause tissue death around the hip and further damage to the patient’s entire body.
Additional complications may occur due to the heavily-criticized metal on metal design of the implant. This design has been criticized due to the metal femoral head and the metal femoral neck rub against each other while in use. This movement can then lead to corrosion of the implant components, ultimately leading to corrective surgeries. Over time, the corrosion of the metal shards by pressing against each other can lead to the metal coursing into the blood stream of the patient who was implanted with a possibly defective Stryker LFIT COCR V40 femoral head. This is life-threatening once metal levels in the blood reach a certain point, leading to possible diagnoses of metallosis or metal poisoning, which can cause tissue death around the hip and further damage to the patient’s entire body.
Common complications could include:
- The site on the hip of the LFIT V40 device becoming swollen or painful
- Surgical complications, causing prolonged time and the possibility of additional surgeries
- Decreased stability and potential weakness in both the joints of the hip and legs
- Pain resulting from the LFIT V40 device loosening
- Tissue around the LFIT V40 becoming irritated or infected
Some other side effects include:
- Infection
- Severe pain
- Dislocation
- Loss of mobility
- Inflammation
- Need for revision surgery
- Fractures
- Dissociation
Stryker LFIT COCR V40 Femoral Head Recall

On August 24, 2016, the Stryker LFIT COCR V40 femoral head was recalled in Canada due to safety concerns. Health Canada’s website stated that the recall was due to higher than expected taper lock failure associated with the femoral head component of the implant. Read more here.
On September 27, 2016, Australian health authorities issued a “Hazard Alert” over the risk of injuries relating to the Stryker LFIT V40 femoral head hip implants citing the same dangers and warning consumers and health professionals about the higher than expected incidence of failures and injuries relating to Stryker LFIT V40 hip implant components. Read that alert here.
It is unknown at this time whether the U.S. Food & Drug Administration (FDA) will issue similar warnings to those in Australia, or require Stryker to recall the LFIT V40 femoral head. We here at d’Oliveira & Associates will continue to monitor the situation, and will report on any update necessary.
Contact a Stryker LFIT COCR V40 Femoral Head Recall Lawyer
 If you or a loved one has suffered because of the Stryker LFIT COCR V40 Femoral Head, then you may want to talk to a personal injury attorney. You may be entitled to damages for your medical bills, lost income, pain and suffering, or other injuries. Our law firm works with experienced Stryker Hip Implant lawyers across the country, who are now filing Stryker LFIT COCR V40 Femoral Head lawsuits. And, there are no attorneys’ fees until you receive a settlement or award. Please call us at 1-800-992-6878 or fill out an online contact form in confidence.
If you or a loved one has suffered because of the Stryker LFIT COCR V40 Femoral Head, then you may want to talk to a personal injury attorney. You may be entitled to damages for your medical bills, lost income, pain and suffering, or other injuries. Our law firm works with experienced Stryker Hip Implant lawyers across the country, who are now filing Stryker LFIT COCR V40 Femoral Head lawsuits. And, there are no attorneys’ fees until you receive a settlement or award. Please call us at 1-800-992-6878 or fill out an online contact form in confidence.
