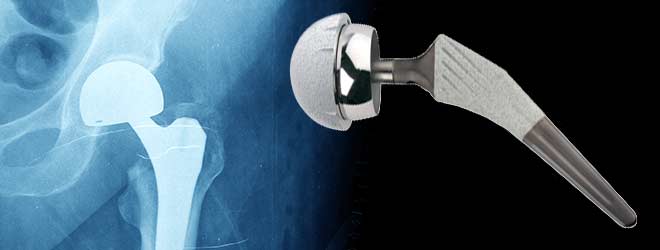
Hip replacement surgery itself is a painful, time-consuming process. The decision to undergo this surgery is not an easy one. The procedure usually requires months of recovery time in order for a patient to return to normal activities. Patients who have made this difficult decision have relied both on the hip replacement devices that will become a part of their body and on their physicians’ discretion. Patients often have supposed that, although the hip replacement process will be painful at first, in the long run, will make them better off.
Despite many individual’s reasonable belief that hip replacement surgery is the best decision, disturbing reports recently have emerged, indicating that a specific type of hip replacement system may be unsafe. These reports indicate that several individuals who have had defective hip replacement devices implanted during their surgery will now have to undergo additional surgeries in order to correct the dangers arising from the defective devices, manufactured by DePuy Orthopaedics.
Total hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant. This joint replacement orthopaedic surgery is performed for various reasons, including hip fracture treatment, or to reduce arthritis pain.
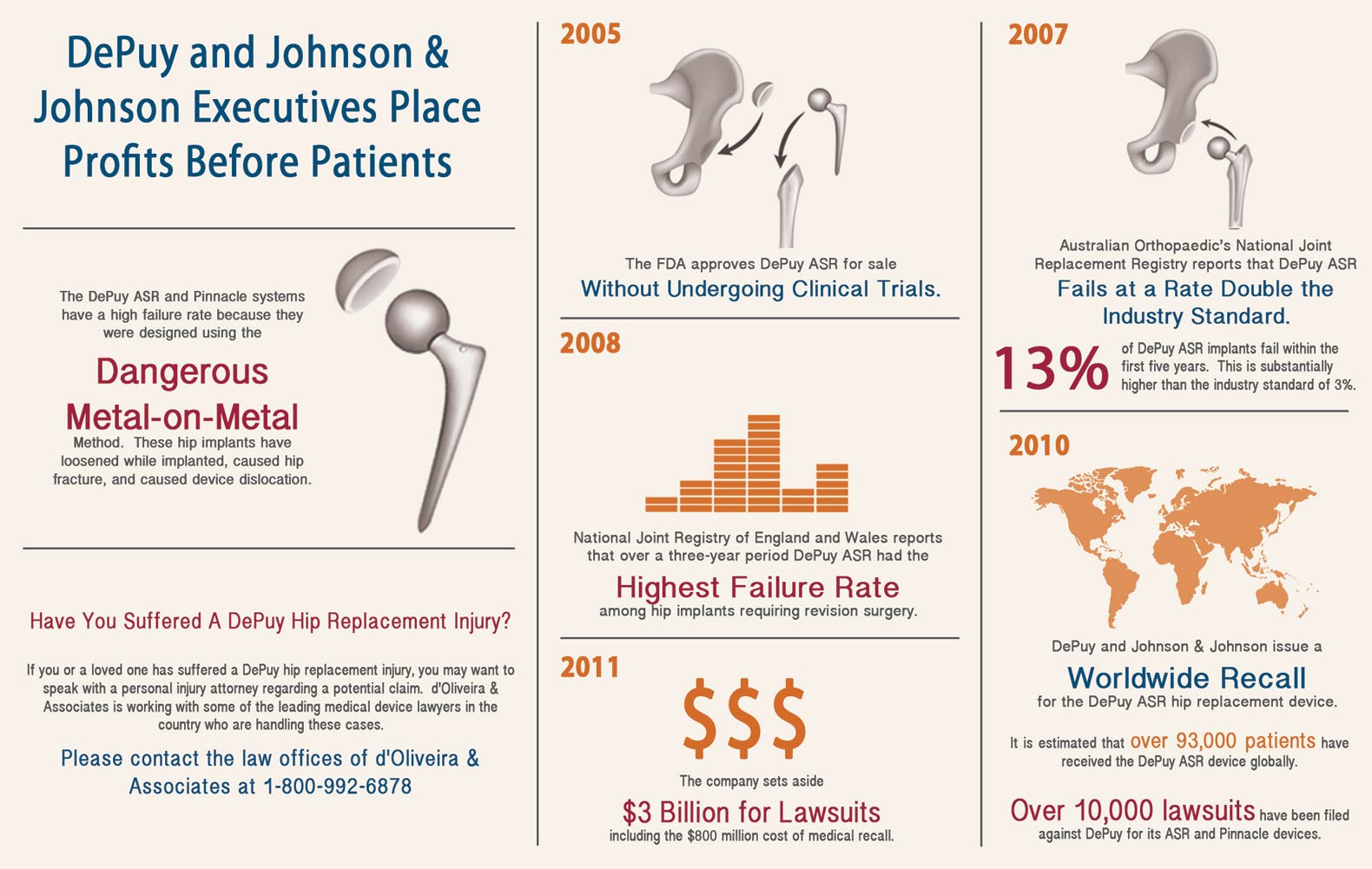
The DePuy ASR hip replacement system has been identified as posing serious risks to implant recipients, including hip failure and dispersal of metallic debris in patients’ bodies. About 93,000 people worldwide have used the devices that are now being recalled because of their high failure rate and, in consequence, the need for repeat corrective surgeries. Following DePuy Orthopaedics’ (a subsidiary of Johnson and Johnson) market withdrawal of the DePuy ASR hip replacement system, and warning to clinicians concerning failure rate information, the U.S. Food and Drug Administration (FDA) has designated the manufacturer’s actions as a Class 2 medical implant recall.
The DePuy ASR is a metal-on-metal hip replacement system. Metal-on-metal hip implants increasingly have been associated with various health risks because they are known to disperse metal particles into the body. This shedding of particles can cause tissue damage and deterioration, inflammatory reactions, and can lead to bone loss. Studies have shown that approximately 12 to 13 percent of patients that have undergone hip replacement surgery with DePuy devices will need corrective or replacement surgery within five years to remedy dangers caused by the metal debris.
DePuy should be reprehended with regard to its actions and irresponsible conduct in this matter. In fact, DePuy had tried to downplay the seriousness of the dangers associated with its devices by initially indicating that the market withdrawal of its products was due to slow sales rates, rather than critical safety issues.
The FDA needs to take more action to ensure that large manufacturers, like DePuy, cannot misrepresent the safety of their products at the expense of a consumer who lacks certain vital informational channels. DePuy should have taken responsibility for its products’ failure early on, rather than misleading the public. Until the FDA betters its process for review of medical manufacture’s devices, and until large manufacturers are reprimanded for their irresponsibility, the average consumer will continue to suffer.