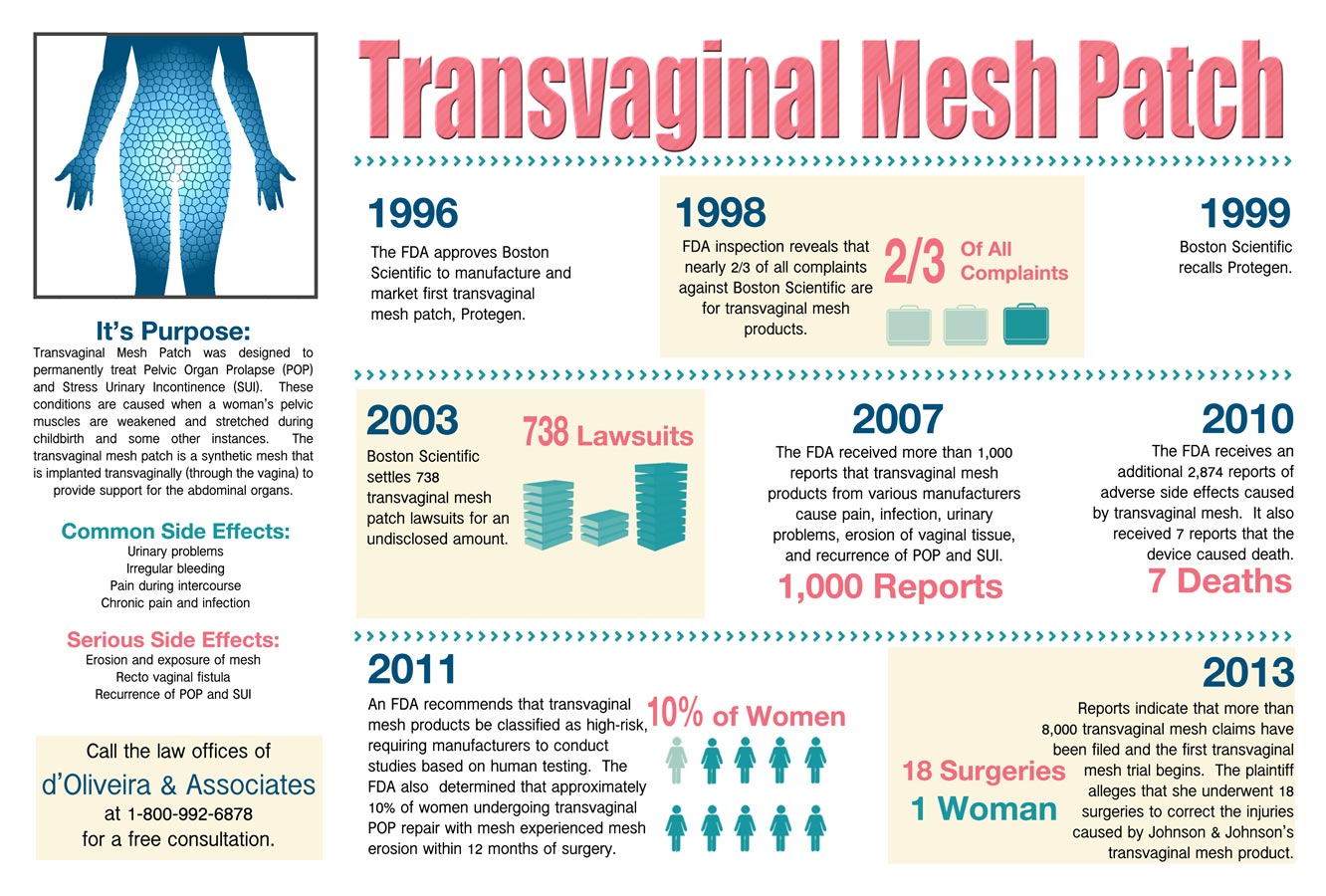
Gynecare Prolift is a vaginal mesh device. It is implanted in women for the treatment of Stress Urinary Incontinence (SUI) and Pelvic Organ Prolapse (POP) caused by the natural weakening of the muscles that support the pelvic organs. Most transvaginal mesh products are approved through The U.S. Food and Drug Administration’s (FDA) 510(k) fast-track approval process, which grants pre-market safety testing to a product that is similar in design to a previously-approved device.
How did Johnson & Johnson Seek to Bypass the FDA
Johnson & Johnson introduced Gynecare Prolift to the market in 2005 and continued to sell it for 2 years without ever having the transvaginal mesh device cleared by the FDA. The company was under the belief that the product was too similar to another product that it was also selling, that it was not required to approach the FDA for approval. Johnson & Johnson was under the false impression that because the Gynecare Prolift was so similar to an another approved device, that it could employ the 501(k) fast track approval. However, although the 501(k) allows for expedited approval, Johnson & Johnson was still required to approach the FDA to show that its Gynecare Prolift was substantially similar to other approved products before marketing it.
 The FDA, upon learning about Johnson & Johnson’s unauthorized marketing, warned the company that the device could not be marketed until the FDA approved it and directed it to halt sales and to provide information on 16 potential deficiencies with the product. The product was approved nine months after the warning. However, during this period, Johnson & Johnson once again ignored the FDA and allowed Gynecare Prolift remained in the market. Despite this, FDA did not sanction the company for failing to withdraw the product from the market despite its warnings. Complaints were also received claiming that the mesh caused pain, scarring, nerve damage and organ perforation – the company currently faces 1,400 lawsuits regarding these injuries. This is not the first occasion J&J have come under fire from the FDA regarding Gynecare Prolift either.
The FDA, upon learning about Johnson & Johnson’s unauthorized marketing, warned the company that the device could not be marketed until the FDA approved it and directed it to halt sales and to provide information on 16 potential deficiencies with the product. The product was approved nine months after the warning. However, during this period, Johnson & Johnson once again ignored the FDA and allowed Gynecare Prolift remained in the market. Despite this, FDA did not sanction the company for failing to withdraw the product from the market despite its warnings. Complaints were also received claiming that the mesh caused pain, scarring, nerve damage and organ perforation – the company currently faces 1,400 lawsuits regarding these injuries. This is not the first occasion J&J have come under fire from the FDA regarding Gynecare Prolift either.
The Importance of FDA Approval
 The FDA asserts that Federal Law requires drugs and medical devices to be approved before being marketed in the United States because there are serious concerns that drugs marketed without approval may not meet the modern standards for safety, quality, effectiveness, and may also lack adequate warnings and labels. This ensures that physicians and healthcare professionals are well equipped with information regarding that instructions and side effects of the products and drugs they are recommending to patients.
The FDA asserts that Federal Law requires drugs and medical devices to be approved before being marketed in the United States because there are serious concerns that drugs marketed without approval may not meet the modern standards for safety, quality, effectiveness, and may also lack adequate warnings and labels. This ensures that physicians and healthcare professionals are well equipped with information regarding that instructions and side effects of the products and drugs they are recommending to patients.
Have You Been Injured?
 If you have suffered any injury or complication as a result of Gynecare ProLift transvaginal mesh implant, you have the right to seek compensation from the manufacturer and marketer of the product. d’Oliveira & Associates represents individuals who have been injured by the use of a product or device that was marketed before FDA approval. Please contact the lawyers in RI at the offices of d’Oliveira & Associates at 1-800-992-6878 or fill out a contact form for a free legal consultation.
If you have suffered any injury or complication as a result of Gynecare ProLift transvaginal mesh implant, you have the right to seek compensation from the manufacturer and marketer of the product. d’Oliveira & Associates represents individuals who have been injured by the use of a product or device that was marketed before FDA approval. Please contact the lawyers in RI at the offices of d’Oliveira & Associates at 1-800-992-6878 or fill out a contact form for a free legal consultation.
155 North Main St.
Attleboro, MA 02703
(508) 223-1133


