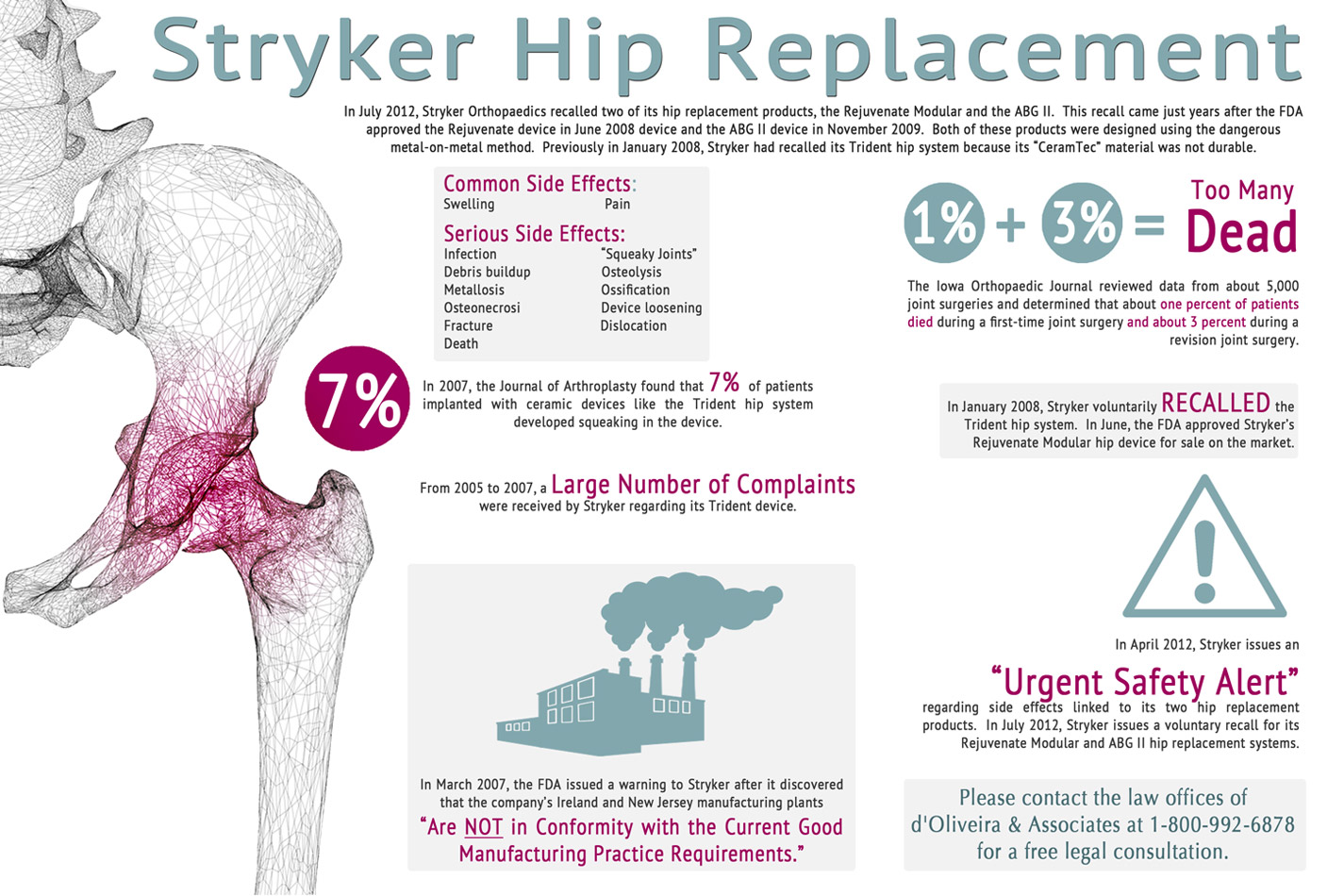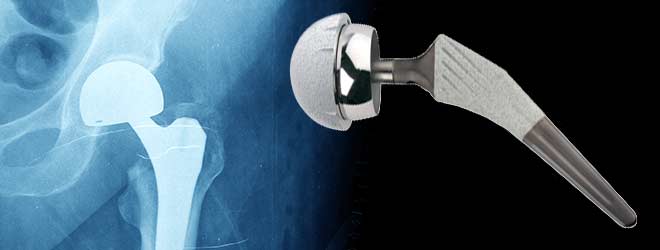
On July 6, 2012, Stryker Howmedica Corporation released an announcement stating that the company was voluntarily recalling two medical device products from the Trident line. These two products that were recalled were the Stryker Rejuvenate and the Stryker AGBII Modular-Neck-Hip Implant. These two devices are affected by corrosion and other problems after being implanted in the body. This corrosion creates risks of serious infection that can create very severe health problems. The possible serious health defects that could occur as a result of the Stryker products are hip implant failure, corrosion, infections, pain, organ failure and death, all of which result from this metal on metal structure.
The Stryker Hip Recall
Stryker first began receiving these complaints around January of 2005 that patients implanted with the Stryker device were experiencing extreme pain in the area where the device was implanted. Also, there were reports from patients that the joint that was replaced squeaked when they walked or moved it. The Stryker Hip Implant was creating extreme pain and discomfort in the patient and made it difficult for the person to walk or sit properly. The joint would wear unevenly and also when the device was fitted improperly it would break off or lead to bone fractures in the body of the patient.

Although the Food and Drug Administration did a prompt investigation of the Stryker product lines, the problems that they found were related to quality control. The quality control problems that they found were related to the presence of a disease causing bacteria in the New Jersey manufacturing plant. The Food and Drug Administration issued a prompt warning to Stryker to fix the problems that were articulated or the Food and Drug Administration would revoke their right to produce the product, seize their property and take any government contracts away.
The question that remains is, if you were harmed or implanted with this device, what recourse do you have? If the Stryker Hip Implant harmed you, you should first contact your surgeon. Your surgeon will be able to better explain your option medically. Many patients will have to get replacement parts for their Stryker Hip Implant. This requires another surgery, more recovery time, more pain and physical therapy. Once you have spoken with your surgeon, you should then contact an experienced RI defective medical device attorney. An experienced RI defective medical device attorney can help you to make important personal decisions that will affect the rest of your life.
Contact the Law Offices of d’Oliveira & Associates
 If you or a loved one has been injured by a defective Stryker Hip Implant component you may want to speak with a Stryker Hip Implant lawyer concerning your rights. A dangerous drug lawyer familiar with new developments in the investigation of the Stryker Hip recall may be able to help you make important personal and legal decisions. d’Oliveira & Associates is working with some of the leading lawyers in the country who are handling these cases.
If you or a loved one has been injured by a defective Stryker Hip Implant component you may want to speak with a Stryker Hip Implant lawyer concerning your rights. A dangerous drug lawyer familiar with new developments in the investigation of the Stryker Hip recall may be able to help you make important personal and legal decisions. d’Oliveira & Associates is working with some of the leading lawyers in the country who are handling these cases.
Please contact the law offices of d’Oliveira & Associates at 1-800-992-6878 or fill out a contact form for a free legal consultation.