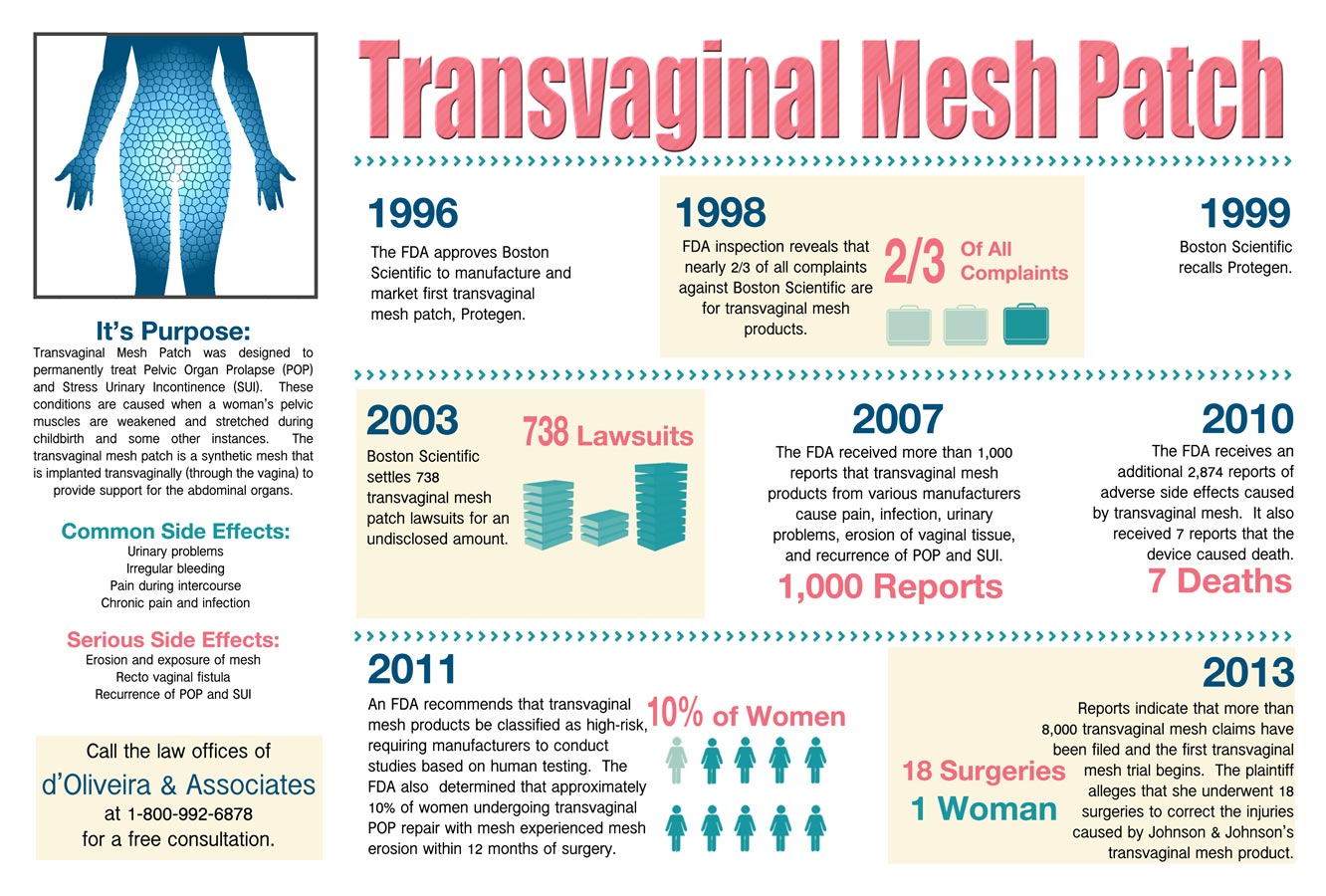
A surgical mesh patch is a permanent synthetic implant designed and used for the repair of ventral or incisional hernias that result from the thinning and stretching scar tissues that typically form after surgeries. It is placed behind the hernia through a small incision, treating Pelvic Organ Prolapse (POP) and Stress Urinary Incontinence (SUI). Among the major manufacturers of surgical mesh patches are the American Medical Systems, Boston Scientific, and Johnson & Johnson. However, despite the common use of these devices, users have increasingly complained about the mesh patch causing severe pain, and its use has been linked infections, scarring, and organ perforation. For example, the Gynecare Prolift, a transvaginal mesh patch manufactured by Johnson & Johnson has been cited in nearly 1,400 claims and lawsuits regarding those side effects.
FDA Issues Recall to Warn Patients and Doctors
The U.S. Food and Drug Administration (FDA) has issued a recall notice to patients who have undergone the surgical procedure to have the trasvaginal mesh patches implanted, urging them to seek the guidance of medical experts immediately. The recall is due, in large part, to the increasing reports of the mesh patch’s plastic recoil ring breaking under stress and that break causing scarring of tissues and organ perforation. That in turn has been linked to subsequent infections to the scarred area. Included in the FDA recall was a recommendation for the medical experts to seriously consider other treatment options and to ensure that patients are properly informed of potential side effects and complications from surgical mesh. While some patients have been informed about the recall, many of those who have already undergone surgery may not be aware of the consequences, or that their infections have been caused by the surgical mesh patch.
Transvaginal Mesh Patch Side Effects
Scientific data that are available show that the transvaginal mesh patch creates significantly more risks than are found in non-mesh surgery. Reported instances include, the memory recoil ring having moved through the patient’s abdominal wall, scarring the tissues and this causing infections. Others have suffered bowel perforation problems and chronic intestinal fistulae. Some women also complained of bulging, pressure, or heavy sensation in the vagina, difficulty in urination, urine leakage and urinary frequency. In one alarming case, a patient has died after developing blood clots, septic shock and acute heart attack from a surgery caused by bowel perforation. Because the growing list of complaints and side effects, the FDA has ordered the recall until it can properly investigate the devices. In 2008, the FDA issued a warning that risks are associated with transvaginal meshes but stressed that those complications are rare. However, now the FDA has acknowledged its error after receiving reports that in a five year period, there were over 4,000 complaints of associated with the mesh patches and several deaths.
Have You Suffered Complications From The Use of Tranvaginal Mesh?
 If you have suffered any injury or complication as a result of a transvaginal mesh implant, you have the right to seek compensation from the manufacturer and marketer of the product. d’Oliveira & Associates represents individuals who have been injured by the use of a product or device that was marketed before FDA approval. Please contact the law offices of d’Oliveira & Associates at 1-800-992-6878 or fill out a contact form for a free legal consultation.
If you have suffered any injury or complication as a result of a transvaginal mesh implant, you have the right to seek compensation from the manufacturer and marketer of the product. d’Oliveira & Associates represents individuals who have been injured by the use of a product or device that was marketed before FDA approval. Please contact the law offices of d’Oliveira & Associates at 1-800-992-6878 or fill out a contact form for a free legal consultation.