
If you or a loved one has suffered infections or other serious injuries as a result of the Physiomesh Patch, you may be entitled to compensation. We are presently working with experienced Physiomesh Patch lawyers across the country, who can file a lawsuit on your behalf, and charge no fee until you receive an award or settlement. Please contact us toll free at any time a 1-800-992-6878, or visit us online for a free consultation.
Have You Suffered Serious Complications From an Implanted Physiomesh Patch?
Call Us Now For a Free Case Evaluation!
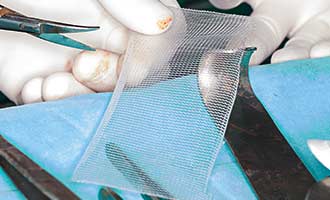
What is a Physiomesh Patch?
Physiomesh is hernia patch that is made from flexible, plastic filaments that are used to repair hernia and reinforce weaknesses in the abdominal wall, which is a site of many hernias. Physiomesh was created by Ethicon, which is a subsidiary of Johnson & Johnson. The patch has been on the United States market since 2010.
What is the Problem with Physiomesh Patches?
It has been discovered by two well-respected, large sample sized German and Danish studies that higher rates of hernia recurrences, complications and accompanying surgeries are found in those patients who are inserted the Physiomesh Patch when compared to similar hernia patches.
What Are Some of the Physiomesh Patch Complication Side Effects?
- Pain
- Infection
- Vomiting
- Fever
- Hernia recurrence
- Adhesion
- Intestinal blockage
- Surgery
- Mesh migration
- Mesh shrinkage (contraction)
Physiomesh Lawsuits

- September, 2016: A lawsuit was filed against Ethicon by a Florida woman who required extensive surgery to remove the Physiomesh that broke off and grew into her intestines and had begun to cause serious, and life-endangering health problems. Read the complaint here (PDF).
- April, 2016: A lawsuit was filed in the United States District Court for the Southern District of Illinois that stated that the victim was diagnosed with a serious infection in and around where the Physiomesh was placed. This infection required surgery and the victim now continues to suffer from health complications. The trial is set to begin in January, 2018, unless there is a settlement. Read the complaint to the court here (PDF).
Related Medical Studies
- August, 2016: The World Journal of Hernia and Abdominal Wall Surgery published a study in which Physiomesh was compared to two other hernia mesh products. The Physiomesh experiment resulted in the development of greater complications when using Physiomesh compared to other hernia mesh products.
- June, 2016: Surgical Endoscopy published a study looking at several methods utilized to close a hernia defect with Physiomesh. It was found that numerous patients experienced hernia recurrence, irrespective of the technique applied.
- March, 2016: Surgical Endoscopy published a study in which 25 patients were inserted with Physiomesh were compared to 25 patients inserted with another mesh patch. Within six months of the surgery, 20% of those patients that were inserted with Physiomesh had experienced some type hernia recurrence. None of those implanted with the other patch had experienced any type of hernia recurrence.
- August, 2015: Surgical Endoscopy published a study in which Physiomesh was compared to two other new hernia mesh products. Physiomesh showed significantly success rates than other mesh patches.
What Has the FDA Done and What Are They Doing Now?
Because of the amount of similar, or like-wise products in the medical market that have been approved by the FDA, the Physiomesh Patch did not have to be approved with new safety studies. This process is known in the medical industry as a 510(k) application with the FDA. This means that the Physiomesh Patch entered the marketplace with little to no clinical trials completed to see the exact safety of the product.
Physiomesh Patch Recalls
Ethicon, the creator of the patch, voluntarily recalled the Physiomesh Patch on May 25, 2016 based on the findings of both the German and Danish studies. The company then sent out an urgent field safety notice to hospitals and chiefs of surgery throughout the country. Read the entire field safety notice here.
However, it is unknown at this time whether the U.S. Food & Drug Administration (FDA) will issue similar warnings to those of Ethicon. We here at d’Oliveira & Associates will continue to monitor the situation, and will report on any update necessary.
Contact a Physiomesh Patch Lawyer

If you or a loved one has suffered because of the Physiomesh Patch, then you may want to talk to a personal injury attorney. You may be entitled to damages for your medical bills, lost income, pain and suffering, or other injuries. Our law firm works with experienced Physiomesh Patch lawyers across the country, who are now filing Physiomesh Patch lawsuits. And, there are no attorneys’ fees until you receive a settlement or award. Please call us at 1-800-992-6878 or fill out an online contact form in confidence.

