
The HIV drug Truvada and other TDF Drugs cause side effects that include bone disease (causing broken bones) and kidney damage, among others. You may be entitled to compensation for medical bills, lost wages, and pain and suffering, among other damages. We are currently working with experienced Truvada lawyers across the country. They charge no fee unless you get an award or settlement. For a free (no obligation) case evaluation, call our toll-free number 24/7 at 1-800-992-6878 or fill out an online contact form.
Have You Sustained Injuries After Taking Truvada or Other TDF Drugs?
You May Have a Claim.
What Are TDF Drugs?
 Tenofovir Disproxil Fumarate (TDF) is a category of drugs specifically used to treat the symptoms and progression of HIV/AIDS. The original intention for these types of drugs was to only be used on people who are currently infected with the virus to help slow down the progression. Now, the use has expanded to include preventative measures for those who are at a high risk of contracting the virus.
Tenofovir Disproxil Fumarate (TDF) is a category of drugs specifically used to treat the symptoms and progression of HIV/AIDS. The original intention for these types of drugs was to only be used on people who are currently infected with the virus to help slow down the progression. Now, the use has expanded to include preventative measures for those who are at a high risk of contracting the virus.
The drugs that fall under the TDF category include:
- Truvada
- Atripla
- Viread
- Stribild
- Complera
What Is Truvada?
 Truvada is categorized as a TDF drug for the treatment and prevention of HIV/AIDS. In August of 2004, the U.S. Food and Drug Administration (FDA) approved the use of Truvada for only treating patients who currently have HIV. The producer of the drug, Gilead Sciences, Inc., then worked on getting the same drug approved as preventative action for people with a high risk of contracting HIV.
Truvada is categorized as a TDF drug for the treatment and prevention of HIV/AIDS. In August of 2004, the U.S. Food and Drug Administration (FDA) approved the use of Truvada for only treating patients who currently have HIV. The producer of the drug, Gilead Sciences, Inc., then worked on getting the same drug approved as preventative action for people with a high risk of contracting HIV.
In 2012, Truvada was also approved to be used a contraceptive for HIV/AIDS. In order to prevent the disease in uninfected people, the drug blocks the virus from attaching onto healthy cells and spreading. Unfortunately, the drug is only really effective if taken consistently each day. If you miss a few doses, it is likely to be much less effective. Truvada is not a cure for HIV/AIDS but rather just reduces the progression of the virus.
What Is HIV/AIDS?
 HIV is a virus known as human immunodeficiency virus. The virus weakens the body’s immune system by destroying the cells that help protect against diseases and infections making your body almost defenseless.
HIV is a virus known as human immunodeficiency virus. The virus weakens the body’s immune system by destroying the cells that help protect against diseases and infections making your body almost defenseless.
Meanwhile, AIDS is a condition known as acquired immunodeficiency syndrome. The condition is also known as stage 3 HIV because contracting HIV can lead to the development of AIDS. HIV damages the immune system to the point where any infection is hard for the body to fight. This is when HIV turns into AIDS which can lead to deadly infections, such as tuberculosis, pneumonia, and cancer.
Unfortunately, there is no cure yet for HIV/AIDS but the TDF drugs are the newest innovation to help those suffering from the disease feel some relief and also by protecting their loved ones from contracting the disease. AIDS is the actual condition that results from contracting the HIV virus. People are usually diagnosed with AIDS once the virus has sufficiently depleted the body’s immune system.
Why Are TDF Drugs Considered Dangerous?
When the news broke that a new drug was created to help treat HIV/AIDS, the world was so excited. Now, after years of use, patients are reporting serious and deadly side effects. The two most concerning side effects include kidney injuries and bone density injuries. Below are a list of each of the types of injuries and side effects that are associated with them.
What Type of Bone Injuries Can Truvada Cause?
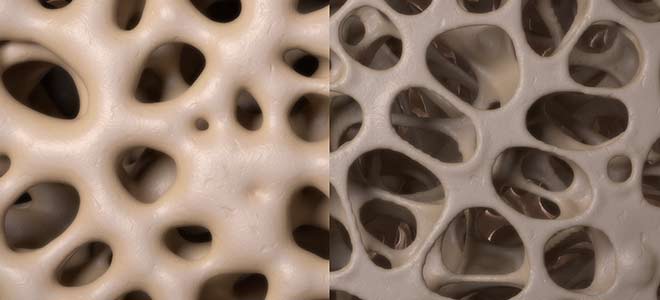
Truvada has been linked to a decrease in bone mineral density (BMD). Some may think well having HIV will generally weaken the entire body’s system so it could just be a result of virus. Unfortunately, a study was conducted of non-HIV infected individuals who used Truvada for preventative measures resulted in lower bone density than normal. Bone density peaks around your early twenties and will continue to slowly decline for the rest of your life. The side effects of Truvada rapidly increase this progress lowering the mass of the bones which results in serious bone injuries.
Types of Bone Injuries:
- Ankle Fractures
- Bone Fractures
- Demineralization of Bones
- Femur Fractures
- Finger Fractures
- Hip Fractures
- Jaw Fractures
- Knee Fractures
- Leg Fractures
- Low Bone Density
- Osteomalacia
- Osteopenia
- Osteoporosis
- Shoulder Fractures
- Spine Curvature
- Spine Fractures
- Toe Fractures
- Weakness of Bones
- Wrist Fractures
What Types of Kidney Injuries Can Truvada Cause?
 Similar to the bone injuries, Truvada has also been linked to an increased risk of damage to the kidneys. For those already infected with HIV, damage to the kidneys can have devastating results. Even those taking Truvada as a preventative measure have reported serious kidney injuries. Kidneys are the sorters of our bodies. They filter our blood and absorb the necessary helpful chemicals. When a drug like Truvada is sorted through the kidneys, it can get built up and damage the kidneys’ function entirely.
Similar to the bone injuries, Truvada has also been linked to an increased risk of damage to the kidneys. For those already infected with HIV, damage to the kidneys can have devastating results. Even those taking Truvada as a preventative measure have reported serious kidney injuries. Kidneys are the sorters of our bodies. They filter our blood and absorb the necessary helpful chemicals. When a drug like Truvada is sorted through the kidneys, it can get built up and damage the kidneys’ function entirely.
Types of Kidney Injuries:
- Acute Renal Failure
- Acute Tubular Necrosis
- Chronic Kidney Disease
- End Stage Renal Failure
- Fanconi Syndrome
- High Levels of Creatine
- Hypophosphatemia
- Kidney Damage
- Kidney Dysfunction
- Kidney Failure
- Nephritis
- Nephrogenic Diabetes Insipidus
- Pain in Kidneys and Lower Back
- Polyuria
- Proteinuria
- Proximal Tubulopathy
- Renal Failure
- Serum Creatinine Increase
- Stage 3 Kidney Disease
- Tubular Dysfunction
If you or a loved one has experienced any of these side effects, you may be entitled to compensation for medical bills, lost wages, and pain and suffering, among other loses. You should contact an experienced Truvada lawyer for a case evaluation.
Is Truvada FDA Approved?
 In August of 2004, the U.S. Food and Drug Administration (FDA) approved the use of Truvada to treat HIV-1 infections in adults. Eight years later, in July of 2012, Truvada was also approved by the FDA for the use of reducing the risk of HIV sexual transmission, also known as Pre-exposure prophylaxis (PrEP). Meanwhile, in May of 2018, the FDA also approved Truvada to now be used for PrEP on adolescent patients.
In August of 2004, the U.S. Food and Drug Administration (FDA) approved the use of Truvada to treat HIV-1 infections in adults. Eight years later, in July of 2012, Truvada was also approved by the FDA for the use of reducing the risk of HIV sexual transmission, also known as Pre-exposure prophylaxis (PrEP). Meanwhile, in May of 2018, the FDA also approved Truvada to now be used for PrEP on adolescent patients.
Truvada History Timeline:
- March 15, 2004 – Gilead Sciences, Inc. applies to the U.S. and European Regulatory Authorities for approval of a fixed dose co-formulation of Viread and Emtriva (TDF drugs).
- March 17, 2004 – The FDA grants Gilead priority review for their TDF drugs Viread and Emtriva.
- August 2, 2004 – Truvada is approved by the FDA to treat people who currently test positive for HIV to reduce progression of the virus.
- July 16, 2012 – FDA approved Truvada for the use of PrEP which is the medical terminology of helping prevent the contraction of HIV for those who are at a high risk of getting the virus.
- May 15, 2018 – The FDA then expands its approval of Truvada to use a prevention treatment for youth and young adults.
- May 9, 2019 – Gilead announces they will provide free Truvada for PrEP to comply with the U.S.’s goal to end HIV/AIDS and especially preventing it from spreading.
Who Is Most At-Risk For Suffering the Dangerous Side Effects of TDF Drugs?
Truvada is used to treat both HIV-infected people, as well as, uninfected people, who are at a high risk of contracting the virus. The following categories of people have been labeled as high-risk:
- Sex workers who may be exposed to HIV.
- People who have sexual intercourse without a condom.
- People in a sexual relationship with an HIV positive partner.
What Does Science Say About TDF Drugs?
- U.S. National Library of Medicine National Institutes of Health – Researchers found a 60% increase in fracture risk in HIV-infected individuals when compared to uninfected individuals.
- Journal of the American Society of Nephrology (JASN) – Researchers discovered a link between TDF types of drugs and an increased risk of tubular toxicity.
- The New England Journal of Medicine – This study found a 44% reduction in the likelihood of contracting HIV when using TDF drugs properly.
Contact an Experienced Truvada Lawyer Today!
 If you or a loved one have taken Truvada and experienced serious side effects, you may be entitled to compensation for lost wages, medical bills, and pain and suffering, among other losses. We work with some of the most experienced TDF defective drug lawyers in the country who are ready to talk to you about a potential case. Call us today toll-free 24/7 at 1-800-992-6878 or fill out an online contact form for a free (no obligation) case evaluation.
If you or a loved one have taken Truvada and experienced serious side effects, you may be entitled to compensation for lost wages, medical bills, and pain and suffering, among other losses. We work with some of the most experienced TDF defective drug lawyers in the country who are ready to talk to you about a potential case. Call us today toll-free 24/7 at 1-800-992-6878 or fill out an online contact form for a free (no obligation) case evaluation.
Sources:
