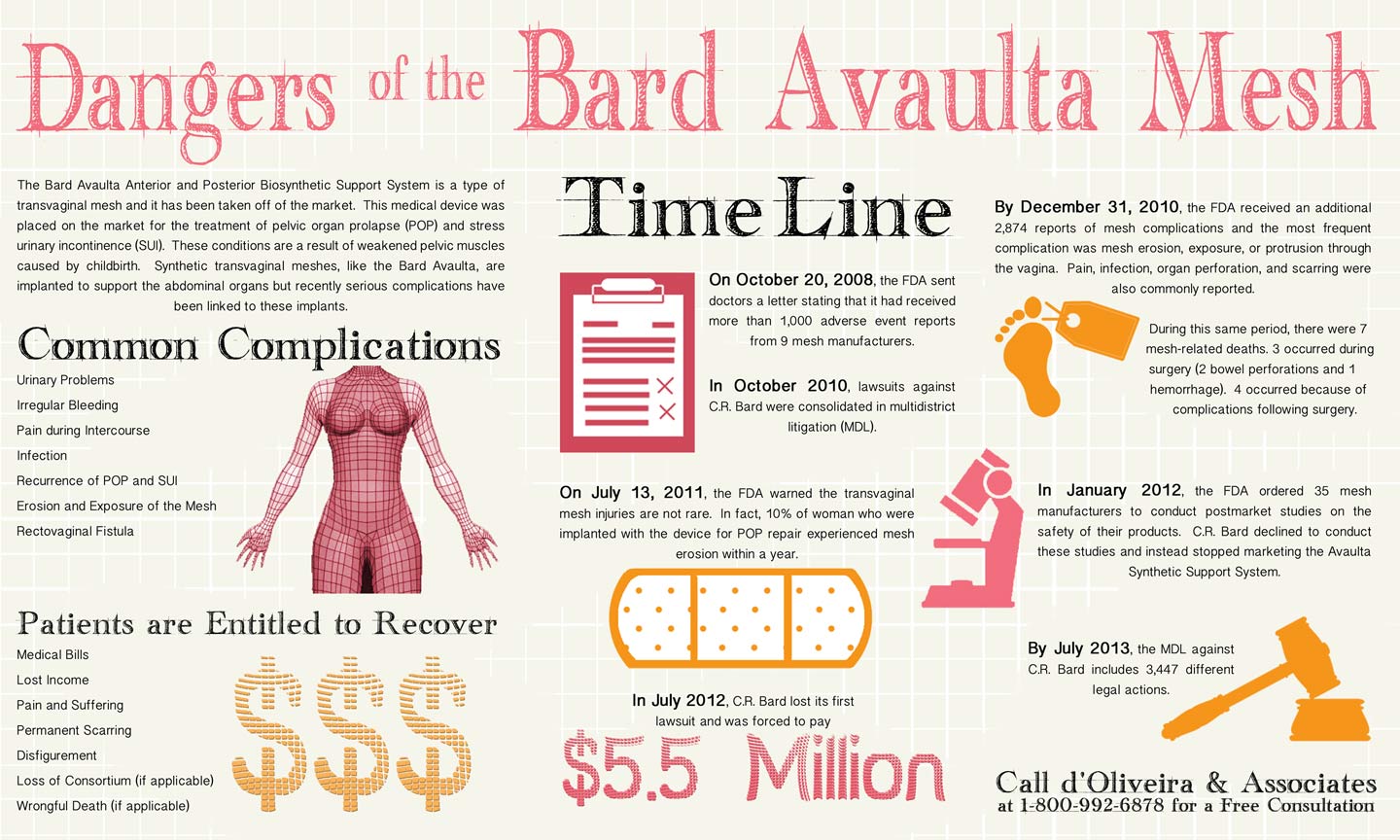
Transvaginal mesh devices were introduced for the treatment of pelvic organ prolapse (POP) and stress urinary incontinence (SUI). In September 2008, the FDA approved the Bard Avaulta Mesh Patch for sale on the market by C.R. Bard. One month after this approval the FDA issued its first major transvaginal mesh warning. The Administration stated that it had received 1,000 adverse event reports regarding these devices. The most common complications were “pain, urinary problems, and recurrence of prolapse and/or incontinence.” Additionally, “in some cases, vaginal scarring and mesh erosion led to significant decrease in patient quality of life due to discomfort and pain” (i). The FDA updated its warnings about these medical devices on July 11, 2011. The Administration stated that “serious complications… are not rare.” Reports showed that up to ten percent of women implanted with the device experience mesh erosion within a year (ii).

In recent years, these injuries have led to thousands of transvaginal mesh lawsuits. There are currently more than 3,000 of these suits filed against C.R. Bard. According to Businessweek, one of the first product liability lawsuits that went to trial has been declared a mistrial (Cisson v. C.R. Bard Inc., 2:11-cv-00195, U.S. District Court, Southern District of West Virginia). Judge Goodwin who presided over the case said that certain evidence had been introduced that should not have, “I don’t think it’s a bell that can be unrung” (iii). C.R. Bard will now have to go through the trial process again and defend its actions in front of a jury.
The Bard Avaulta Infographic provides a timeline following the device’s approval in 2008 to its market withdrawal in 2012 and subsequent legal actions. Women who have been injured because of mesh erosion may be entitled to compensation for medical expenses, lost income, pain and suffering, or other losses. d’Oliveira & Associates has been looking into the safety of these medical devices and allegations of serious complications. The law firm works with some of the more experienced transvaginal mesh lawyers and there are no legal fees until a settlement or award is reached. Contact the firm by calling toll-free at 1-800-992-6878 or fill out an online contact form.
Source:
- (i) FDA Public Health Notification, October 20, 2008.
http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm061976.htm - (ii) FDA Safety Communication, July 13, 2011.
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm - (iii) Businessweek, July 10, 2013.
http://www.businessweek.com/news/2013-07-10/cr-bard-case-over-vaginal-mesh-defects-declared-mistrial-1