
If you or a loved one has suffered serious injuries as a result of being implanted with a Modular Revision Femoral Hip System, you may be entitled to compensation for your injuries. We are currently working with experienced Modular Revision Femoral Hip System lawyers across the country, who are able to file a lawsuit on your behalf, and will not charge a fee until you receive an award or settlement. Please contact us toll free at any time, or visit us online for a free consultation.
Have You Suffered Serious Complications From an Implanted Modular Revision Femoral Hip System?
Call Us Now For a Free Case Evaluation!
What is a Modular Revision Femoral Hip System?
Modular revision femoral hip replacement systems are sets of metal implants that are specially designed to be used in hip replacement revision procedures. These implants are primarily used to fix prior hip replacements that have failed, and in instances where patients have severely damaged hip joints as a result of trauma or degenerative diseases, such as osteoarthritis. Each set is made up of a modular neck, a modular sleeve, and a femoral stem. These pieces are designed to interlock with a liner and another metal component called a femoral head to create one structure connecting the hip joint to the femur bone. This particular model is manufactured by Smith & Nephew, Inc. USA. The two models in this category include the Modular SMF™ and Modular REDAPT™ Revision Femoral Hip Systems.
What is the Problem with Modular Revision Femoral Hip Systems?
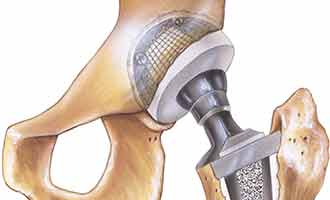 Although thought to be very safe and effective when first approved by the Food and Drug Administration (FDA) in 2009, the Smith & Nephew modular neck hip implant may actually be more dangerous than expected. There have been numerous adverse side effects and complications reported in connection with the Smith & Nephew hip replacement systems. Most of the complaints involve the implants becoming dislocated and wearing down quickly. Another common complaint is metal toxin being released into the patient’s body due to the metal components corroding and rubbing together during normal use. This causes tissue, muscle and bone surrounding the implant site to break down and become infected. Another concern is metal poisoning from the release of toxins into the body, which can cause permanent organ damage.
Although thought to be very safe and effective when first approved by the Food and Drug Administration (FDA) in 2009, the Smith & Nephew modular neck hip implant may actually be more dangerous than expected. There have been numerous adverse side effects and complications reported in connection with the Smith & Nephew hip replacement systems. Most of the complaints involve the implants becoming dislocated and wearing down quickly. Another common complaint is metal toxin being released into the patient’s body due to the metal components corroding and rubbing together during normal use. This causes tissue, muscle and bone surrounding the implant site to break down and become infected. Another concern is metal poisoning from the release of toxins into the body, which can cause permanent organ damage.
The problem points to the design of the Modular SMF™ and Modular REDAPT™ Revision Femoral Hip Systems. They are comprised of metal parts that lock together in order to facilitate movement and stability. Because they are designed to be in frequent motion, the possibility of corrosion and metal on metal contact is very high. The liners placed in between the metal ball and metal stem have been shown to wear down at an accelerated rate, which increases the risk of dislocation and the release of metal toxins. Many patients who have experienced these side effects have had to undergo extensive procedures to remove and replace the revision systems.
What Are Some of the Modular Revision Femoral Hip System Complications?
- Metallosis (metal poisoning)
- Corrosion
- Tissue damage, or death
- Bone loss
- Pseudo-tumor formation
- Severe pain in the hip joint
- Inflammation
- Swelling
- Misalignment or dislocation of the hip joint
- Need for revision surgery
What Has the FDA Done and What Are They Doing Now?
 The FDA issued a Safety Communication on January 17, 2013 warning patients and healthcare providers that metal on metal hip implants carry unique risks in addition to the general risks of all hip implant systems. Specifically, the warning notes that metal on metal implants have a tendency to chip and release metal ions into the body, damaging surrounding tissue and causing other health issues due to high levels of metal in the bloodstream.
The FDA issued a Safety Communication on January 17, 2013 warning patients and healthcare providers that metal on metal hip implants carry unique risks in addition to the general risks of all hip implant systems. Specifically, the warning notes that metal on metal implants have a tendency to chip and release metal ions into the body, damaging surrounding tissue and causing other health issues due to high levels of metal in the bloodstream.- On January 17, 2013 the FDA issued a proposed order that would require manufacturers of metal on metal total hip replacement systems to complete premarket approval (PMA) applications, which would hold manufacturers to higher standards to get approval.
- On July 27, 2012, the FDA held a meeting with the Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee to hear expert clinical opinions on the risks of metal-on-metal hip implant systems.
- Prior to this, the FDA had been concerned about the safety of metal on metal hip implants. After receiving multiple reports of adverse side effects, the FDA ordered manufacturers of metal on metal hip replacement systems to conduct post market surveillance study on May 6, 2011. This will provide additional information to patients and doctors about the safety of these implants, including the effect of metal ions in the blood stream.
Modular Revision Femoral Hip System Recalls
 On November 15, 2016 Smith & Nephew issued a letter voluntarily recalling the modular neck of the Modular SMF™ and Modular REDAPT™ Revision Femoral Hip Systems, which they listed as an Urgent Field Safety Notice and Corrective Action Recall. The medical device manufacturer decided to recall the implant systems due to a higher than expected number of complaints and an ongoing trend of adverse side effects. Smith & Nephew noted the number of adverse events reported were higher than other similar hip implant systems. Specifically cited in the letter was the risk of tissue damage due to metal debris from the implants, which can lead to the need for revision surgery to remove the defective implants.
On November 15, 2016 Smith & Nephew issued a letter voluntarily recalling the modular neck of the Modular SMF™ and Modular REDAPT™ Revision Femoral Hip Systems, which they listed as an Urgent Field Safety Notice and Corrective Action Recall. The medical device manufacturer decided to recall the implant systems due to a higher than expected number of complaints and an ongoing trend of adverse side effects. Smith & Nephew noted the number of adverse events reported were higher than other similar hip implant systems. Specifically cited in the letter was the risk of tissue damage due to metal debris from the implants, which can lead to the need for revision surgery to remove the defective implants.
Contact a Modular Revision Femoral Hip System Lawyer
 If you or a loved one has suffered because of the Modular Revision Femoral Hip System, then you may want to talk to a personal injury attorney. You may be entitled to damages for your medical bills, lost income, pain and suffering, or other injuries. Our law firm works with experienced Modular Revision Femoral Hip System lawyers across the country, who are now filing Modular Revision Femoral Hip System lawsuits. And, there are no attorneys’ fees until you receive a settlement or award. Please call us at 1-800-992-6878 or fill out an online contact form in confidence.
If you or a loved one has suffered because of the Modular Revision Femoral Hip System, then you may want to talk to a personal injury attorney. You may be entitled to damages for your medical bills, lost income, pain and suffering, or other injuries. Our law firm works with experienced Modular Revision Femoral Hip System lawyers across the country, who are now filing Modular Revision Femoral Hip System lawsuits. And, there are no attorneys’ fees until you receive a settlement or award. Please call us at 1-800-992-6878 or fill out an online contact form in confidence.
